What is Concentration Gradient?
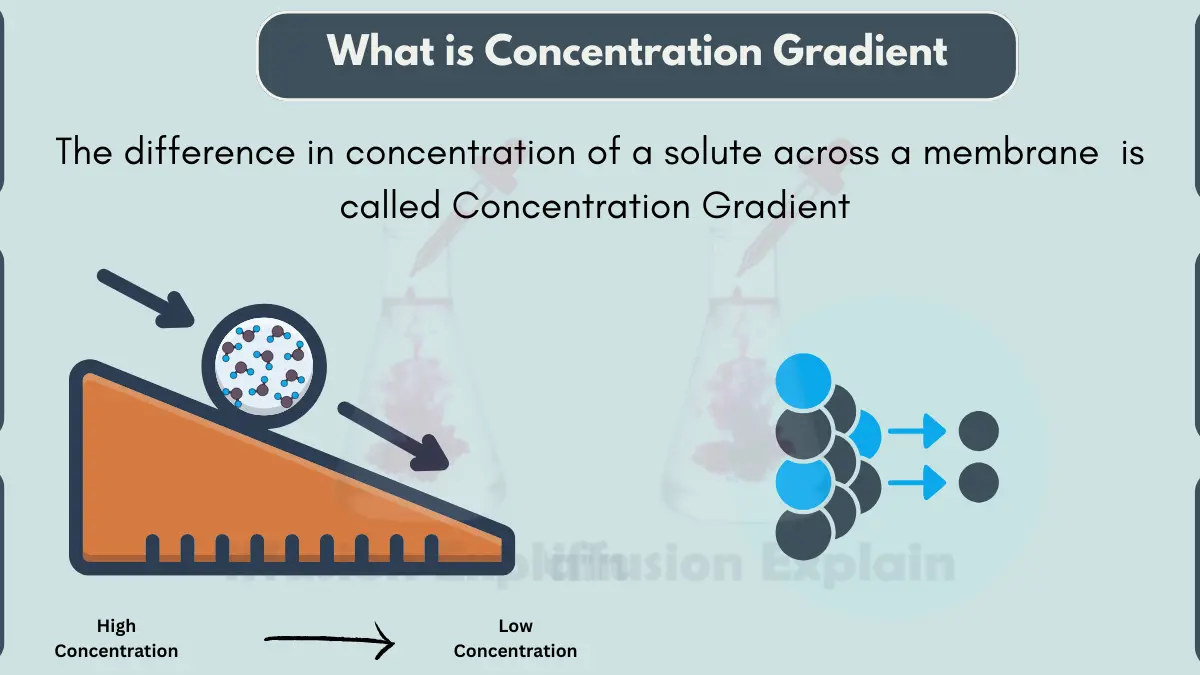
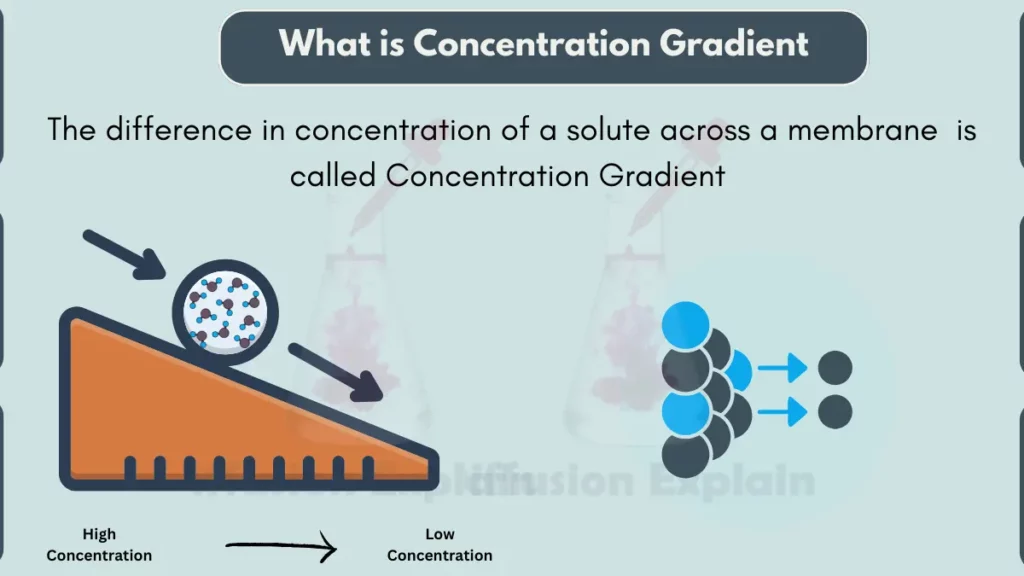
A concentration gradient is the difference in the amount or concentration of something between two points or locations. In simple words, it means that there is more of something in one place compared to another place.
For example, if you put some sugar in a glass of water and don’t stir it, there will be a higher concentration of sugar at the bottom of the glass where the sugar was added and a lower concentration of sugar at the top of the glass. This difference in the amount of sugar from the bottom to the top is called a concentration gradient.
Concentration gradients are important in many natural processes, such as the movement of molecules across cell membranes or the diffusion of gases in the atmosphere. Things tend to move from areas of higher concentration to areas of lower concentration, by following the concentration gradient.
Role of Concentration Gradient in Diffusion
Concentration gradient refers to the difference in the amount or concentration of something between two areas or regions. This concentration difference is the driving force for diffusion to occur.
For example, if you have a lot of a substance like dye or perfume in one area, but very little in another area nearby, there is a concentration gradient between those two areas.
Diffusion is the natural process where particles spread out from an area of high concentration to an area of low concentration. This happens because the particles are constantly moving around randomly and tend to spread out evenly over time.
The concentration gradient acts as the driving force for diffusion to occur. Particles will naturally move from the area of high concentration down the concentration gradient toward the area of low concentration.
For example, if you put a drop of food coloring in water, at first it is very concentrated in just that spot. But over time, the food coloring particles will diffuse and spread out through the whole glass of water, following the concentration gradient, until the color is evenly distributed.
The larger the concentration gradient between two areas, the faster the diffusion will happen to try to even out the difference in concentration between those areas.
Diffusion continues until the concentration gradient has been eliminated and the particles are evenly spread out everywhere with no difference in concentration between regions.
Role of Concentration Gradient in Osmosis
Osmosis is the movement of water molecules across a semi-permeable membrane from a region of higher water concentration (lower solute concentration) to a region of lower water concentration (higher solute concentration).
The concentration gradient that drives osmosis is the difference in solute concentrations between the two solutions on either side of the membrane.
The region with a higher concentration of solute particles (like salt or sugar) has fewer free water molecules. This creates a lower concentration of water compared to the region with fewer solute particles.
Water molecules naturally move from the area of higher water concentration (lower solute) to the area of lower water concentration (higher solute) by osmosis, following this concentration gradient.
The greater the difference in solute concentrations between the two solutions, the steeper the concentration gradient, and the faster the rate of osmosis.
Osmosis continues until the solute concentrations on both sides become equal. It eliminates the concentration gradient. At this equilibrium, the net movement of water stops.
This concentration gradient between solutions of different solute concentrations generates the osmotic pressure that allows osmosis to occur across semi-permeable membranes like those in plant and animal cells. Understanding the difference between diffusion and osmosis helps clarify how concentration gradients drive different types of molecular movement.
Role of Concentration Gradient in Active Transport
In active transport, the concentration gradient plays an indirect but important role:
Active transport is the movement of molecules across a membrane from a region of lower concentration to a region of higher concentration. This goes against the natural concentration gradient, so it requires energy from the cell in the form of ATP.
Even though active transport opposes the concentration gradient, the gradient still determines the direction which molecules need to be transported.
For example, if there is a higher concentration of sodium ions (Na+) outside a cell than inside, the concentration gradient favors sodium moving into the cell by simple diffusion down the gradient.
However, many cells need to pump sodium out against this gradient to maintain proper sodium levels inside the cell. This is active transport.
The role of the concentration gradient is to provide a baseline indicating which direction active transport must occur to move molecules against the natural tendency of diffusion down the gradient.
The greater the difference in concentrations (steeper gradient), the more energy is required for active transport to move molecules from low to high concentration.
Without a concentration gradient, there would be no driving force for diffusion, so active transport would not be needed to move molecules in the opposite direction.
So in summary, while active transport utilizes energy to move molecules against a concentration gradient, that gradient still defines the energetically unfavorable direction that requires active transport mechanisms.
Examples of Concentration Gradient in Active Transport
Here are a few examples of concentration gradient in active transport:
1. Oxygen Diffusion in the Body
Our cells require oxygen for respiration and energy production. The air we breathe in has a higher concentration of oxygen compared to the oxygen levels in our body cells. This creates an oxygen concentration gradient. Oxygen diffuses from lungs (high concentration) into bloodstream and then into the body cells (low concentration).
2. Sugar Dissolving in Water
When you add sugar to a glass of water and let it sit, a concentration gradient is established. Initially, the sugar is concentrated at the bottom of the glass, creating a high sugar concentration in that region. The rest of the water has a low sugar concentration. This gradient causes the sugar molecules to diffuse and spread out evenly throughout the water until the concentration is uniform.
3. Smell of a Fragrance
When you spray a fragrance in one corner of a room, the aroma molecules are highly concentrated in that area. The rest of the room has a lower concentration of those fragrance particles. This creates a concentration gradient, causing the fragrance molecules to diffuse from the high-concentration area (corner) toward the low-concentration areas (rest of the room) until the smell is evenly distributed.
4. Plant Root Absorption
The soil surrounding plant roots often has a higher concentration of water and mineral nutrients compared to the concentrations inside the root cells. This water and nutrient concentration gradient allows the plant roots to absorb water and minerals from the soil through diffusion and osmosis. It follows the gradient from high concentrations in the soil to lower concentrations inside the roots.